We reach more than 65,000 registered users in Dec!! Register Now
Mechanisms of embryonic wound healing - unleashed
- January 05, 2016
- 1444 Views
- 0 Likes
- 0 Comment
The recent inventions in the field of bio-science has reached great heights. Researchers at the University of Toronto (U of T) and the Hospital for Sick Children have found that the process of endocytosis -- how cells "eat" by absorbing molecules -- drives rapid embryonic healing.
The recent inventions in the field of bio-science has reached great heights. Researchers at the University of Toronto (U of T) and the Hospital for Sick Children have found that the process of endocytosis -- how cells "eat" by absorbing molecules -- drives rapid embryonic healing.
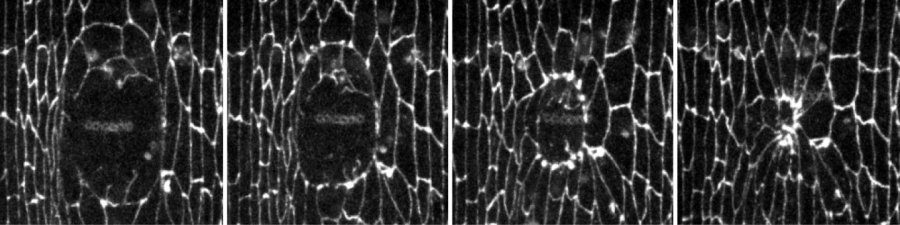
Embryonic epithelia have a remarkable ability to rapidly repair wounds. A supracellular actomyosin cable around the wound coordinates cellular movements and promotes wound closure. Actomyosin cable formation is accompanied by junctional rearrangements at the wound margin.
The process of endocytosis -- how cells 'eat' by absorbing molecules -- drives rapid embryonic healing, scientists have discovered. They suggest the results should be used to design better treatments for wounds in adults.
It's like something out of a science-fiction movie -- time-lapse photography showing how wounds in embryos of fruit flies heal themselves. These were taken using n vivo time-lapse quantitative microscopy. The images are not only real; but also they shed light on ways to improve wound recovery in humans.
"Endocytosis removes the junctions between wounded and non-wounded cells, to allow the non-wounded cells to move and stretch over the wounded area to close the wound," said Miranda Hunter, a PhD candidate in the Department of Cell & Systems Biology and lead author of a research study published in the August 31 issue of the Journal of Cell Biology.
The researchers further found that endocytosis coordinates the movement of the non-wounded cells as they close the wound, by directing the formation of a structure known as a "purse string." The structure assembles around the wound and rapidly contracts to draw the surrounding cells together and close the wound.
"Wounds in embryos heal very quickly and with very little inflammation or scarring," said Hunter. "Our hope is that by understanding how embryos repair wounds, we can translate our understanding into more efficient treatments to induce adult cells to move into the wound area in a coordinated way as embryonic cells do."
The researchers used embryos of fruit flies as a model system for human embryos. Fruit flies are commonly used in genetic studies because they share a surprising number of qualities with humans. Further, they are inexpensive to care for and reproduce rapidly, allowing for several generations to be studied in just a few months.
Hunter's PhD supervisor Rodrigo Fernandez-Gonzalez, a bioengineering specialist in U of T's Department of Cell & Systems Biology and Institute of Biomaterials and Biomedical Engineering, as well as the Developmental and Stem Cell Biology program at the Hospital for Sick Children, said the study offers a range of important new information.
"It shows how cells move in a coordinated manner, which helps us to understand more about processes such as cancer metastasis and embryonic development.”
The scientists found that Blocking endocytosis with pharmacological or genetic approaches disrupted wound repair. The defect in wound closure was accompanied by impaired removal of E-cadherin from the wound edge and defective actomyosin cable assembly. E-cadherin overexpression also resulted in reduced actin accumulation around wounds and slower wound closure.
Their results demonstrate a central role for endocytosis in wound healing and indicate that polarized E-cadherin endocytosis is necessary for actomyosin remodeling during embryonic wound repair.
Source:
http://www.sciencedaily.com/releases/2015/08/150831085105.htm
Journal Reference:
M. V. Hunter, D. M. Lee, T. J. C. Harris, R. Fernandez-Gonzalez. Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair. The Journal of Cell Biology, 2015; DOI: 10.1083/jcb.201501076