We reach more than 65,000 registered users in Dec!! Register Now
SINGAPORE SCIENTISTS DEVELOP GENE-EDITING TECHNOLOGY THAT ELIMINATES EV-A71 RNA VIRUSES
- November 17, 2024
- 2 Views
- 0 Likes
- 0 Comment
SINGAPORE SCIENTISTS DEVELOP GENE-EDITING TECHNOLOGY THAT ELIMINATES EV-A71 RNA VIRUSES
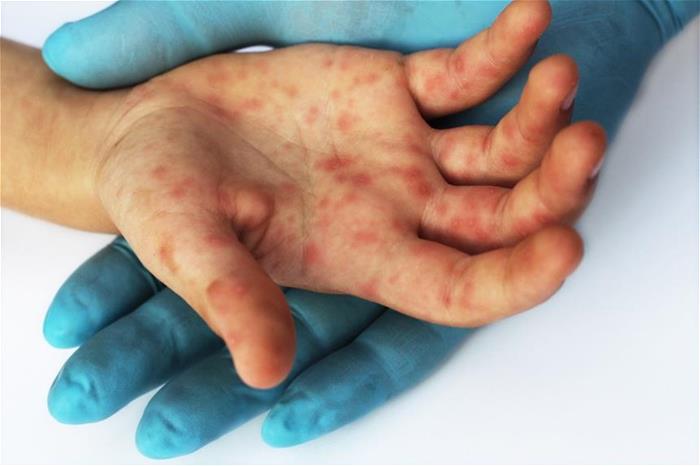 Singapore scientists have developed a CRISPR-Cas13 therapeutic against EV-A71, the RNA virus that causes hand, foot, and mouth disease.1 August 2023 – A team of scientists from A*STAR’s Genome Institute of Singapore (GIS) and the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) has made an important breakthrough in the fight against RNA viruses that cause human diseases and pandemics.
Singapore scientists have developed a CRISPR-Cas13 therapeutic against EV-A71, the RNA virus that causes hand, foot, and mouth disease.1 August 2023 – A team of scientists from A*STAR’s Genome Institute of Singapore (GIS) and the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) has made an important breakthrough in the fight against RNA viruses that cause human diseases and pandemics.Their research shows that the CRISPR-Cas13 editor delivered by adeno-associated virus (AAV) can directly target and eliminate RNA viruses in laboratory models. AAV are delivery vehicles derived from small viruses that naturally infect humans. They are clinically approved for use in gene therapy drugs which are used to treat diseases such as spinal muscular atrophy, Duchenne muscular dystrophy, and haemophilia.
The EV-A71 virus is the cause of the hand, foot, and mouth disease, and in severe cases, can lead to nervous system disease and death. To treat the viral infection, the team turned to CRISPR-Cas13, an RNA-editing technology that alters RNA in a cell.
CRISPR-Cas13 edits RNA and opens therapeutic avenues to a wide range of diseases that are untreatable by the Nobel Prize-winning CRISPR-Cas9, which edits DNA.
CRISPR-Cas13 is programmed by guide RNAs (gRNAs) to target specific RNA sequences. Upon binding to these RNA sequences, the CRISPR-Cas13 cuts the RNA target into pieces, inactivating the RNA. CRISPR-Cas13 could also be utilised for RNA-editing, where a specific RNA sequence is changed to another sequence within the cell.
In this recent work, the team of scientists first developed the Cas13gRNAtor computational programme to design CRISPR gRNAs that cut viral RNA across different viral strains. They show that CRISPR-Cas13 treatment potently reduces viral burden, with less than 0.1% of the viruses remaining in previously infected cells.
Importantly, the research findings show that the AAV-CRISPR-Cas13 therapy clears the EV-A71 infection and prevents organ damage and mortality.
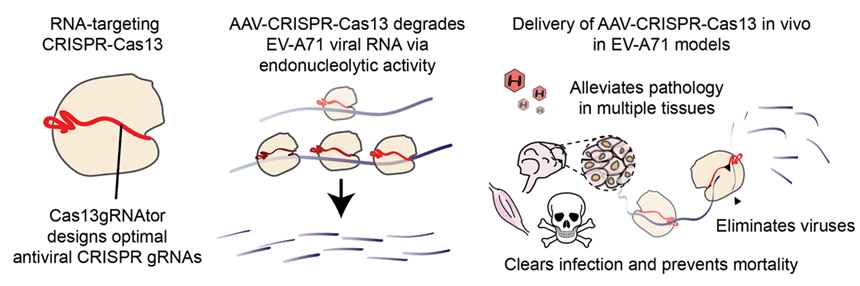 A newly developed antiviral CRISPR-Cas13 therapeutics.“This is a stunning demonstration that one dose of CRISPR-Cas13 can mean a difference between life and death. We are building on this research to develop further life-changing nucleic acid therapeutics.” said Dr Chew Wei Leong, Associate Director and Principal Scientist at A*STAR’s GIS.
A newly developed antiviral CRISPR-Cas13 therapeutics.“This is a stunning demonstration that one dose of CRISPR-Cas13 can mean a difference between life and death. We are building on this research to develop further life-changing nucleic acid therapeutics.” said Dr Chew Wei Leong, Associate Director and Principal Scientist at A*STAR’s GIS.Associate Professor Justin Chu from NUS Medicine’s Department of Microbiology and Immunology and Infectious Diseases Translational Research Programme added, “This amazing study has helped to unlock the new frontiers in antiviral strategies by using AAV-CRISPR-Cas13 to combat human enteroviruses, paving the way for potential therapeutics against viral diseases.”
Professor Liu Jian Jun, Acting Executive Director of A*STAR’s GIS said, “The CRISPR technology allows the rewriting of the genetic code in almost any organism. This joint research with NUS is an extremely important development which can potentially treat many diseases caused by RNA viruses, and open many avenues for further therapeutic solutions.”
These findings demonstrate a therapeutic development pipeline for antiviral AAV-CRISPR-Cas13 against potentially deadly RNA virus infections. Further therapeutic development could bring this technology towards treating human RNA viruses in the clinic. This research was published on 28 June 2023, in eBioMedicine, part of The Lancet Discovery Science.
List of Referenes
- Choong Tat Keng, Thinesshwary Yogarajah, Regina Ching Hua Lee, Irfan Bin Hajis Muhammad, Bing Shao Chia, Suraj Rajan Vasandani, Daryl Shern Lim, Ke Guo, Yi Hao Wong, Chee Keng Mok, Justin Jang Hann Chu, Wei Leong Chew. AAV-CRISPR-Cas13 eliminates human enterovirus and prevents death of infected mice. eBioMedicine, 2023; 93: 104682 DOI: 10.1016/j.ebiom.2023.104682
Cite This Article as
No tags found for this post