We reach more than 65,000 registered users in Dec!! Register Now
Stem and progenitor cell proliferation are independently regulated by cell type-specific cyclinD genes
- July 21, 2025
- 3 Views
- 0 Likes
- 0 Comment
Regeneration and homeostatic turnover of solid tissues depend on the proliferation of symmetrically dividing adult stem cells, which either remain stem cells or differentiate based on their niche position. Here we demonstrate that in zebrafish lateral line sensory organs, stem and progenitor cell proliferation are independently regulated by two cyclinD genes. Loss of ccnd2a impairs stem cell proliferation during development, while loss of ccndx disrupts hair cell progenitor proliferation but allows normal differentiation. Notably, ccnd2a can functionally replace ccndx, indicating that the respective effects of these Cyclins on proliferation are due to cell type-specific expression. However, even though hair cell progenitors differentiate normally in ccndx mutants, they are mispolarized due to hes2 and Emx2 downregulation. Thus, regulated proliferation ensures that equal numbers of hair cells are polarized in opposite directions. Our study reveals cell type-specific roles for cyclinD genes in regulating the different populations of symmetrically dividing cells governing organ development and regeneration, with implications for regenerative medicine and disease.
Tissue turnover and regeneration are essential for organismal function and survival and require the proliferation of stem cells. In most solid tissues, this process relies on symmetrically dividing adult stem cells1. For instance, in the epithelia of the intestine, stomach, esophagus and the skin, stem cells divide symmetrically and—depending on their location in the stem cell niche—the daughter cells either maintain their stem cell characteristics, or if displaced from the niche proceed to differentiate2,3,4,5,6,7,8,9. Stem and progenitor cell proliferation need to be tightly controlled, as misregulation of niche signals or uncontrolled proliferation of stem and daughter cells can lead to serious diseases such as cancer. Despite the importance of symmetrically dividing stem cells and their progeny for tissue maintenance, regeneration and disease, whether their proliferation is differentially regulated has not been explored.
The zebrafish sensory lateral line is an excellent model to study the regulation of sensory organ homeostasis and regeneration at the cellular and molecular level within single cells10,11,12. It consists of clusters of 50–80 cells, called neuromasts that are arranged in lines on the head and along the trunk of the fish (Fig. 1A–C). These neuromasts are deposited during embryonic development by migrating primordia13. Each neuromast contains mechanosensory hair cells surrounded by support cells and peripheral mantle cells (Fig. 1C, D). Hair cells possess a long, microtubule based kinocilium and shorter actin rich stereocilia that are sensitive to water motion (Fig. 1B14).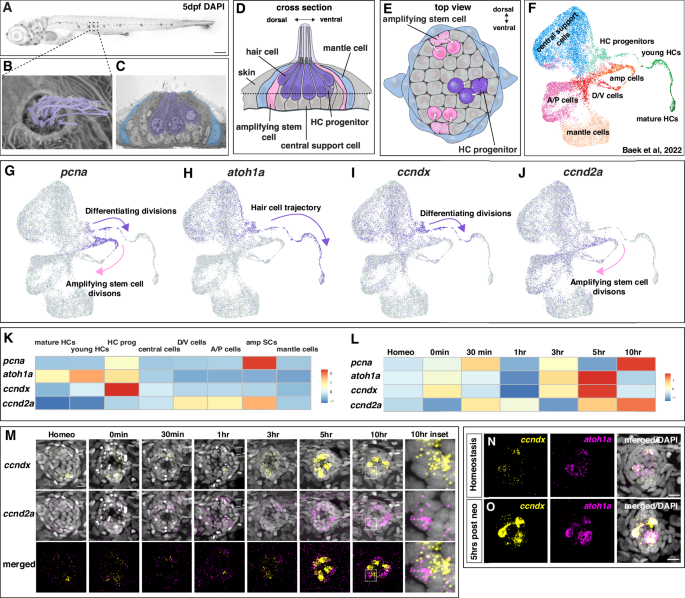 A Representative image of a 5 dpf DAPI-stained zebrafish larva, posterior lateral line neuromast in boxed region. Scale bar = 200 µm. B Scanning electron micrograph of a 5 dpf zebrafish neuromast (dorsal view) with short stereocilia and long kinocilia in purple (adapted from Lush, M.E. and Piotrowski, T. (2014), Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn., 243: 1187-1202. https://doi.org/10.1002/dvdy.24167”10). C Transmission electron micrograph of a transverse section of a 5 dpf neuromast with hair cells in purple and mantle cells in blue. Additional support cells are unlabeled (adapted from Lush, M.E. and Piotrowski, T. (2014), Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn., 243: 1187-1202. https://doi.org/10.1002/dvdy.24167”10). Diagram of a neuromast showing a transverse section (D) and a dorsal view (E). Progenitor cells and hair cells are in purple, amplifying stem cells in the dorsal-ventral poles in pink and mantle cells in blue. F Integrated scRNA-seq UMAP plot of a neuromast regeneration time course (homeostasis, 0 min, 30 min, 1 h, 3 h, 5 h and 10 h after hair cell death; Baek et al., 202212). G–J scRNA-seq Feature Plots (Baek et al., 202212, https://piotrowskilab.shinyapps.io/neuromast_regeneration_scRNAseq_pub_2021/) illustrating gene-specific expression patterns. G pcna labels dividing, differentiating hair cell progenitors (purple arrow) and amplifying stem cells (pink arrow). H atoh1a is expressed in some central cells and marks the lineage from hair cell progenitors to hair cells (purple arrow). I ccndx is expressed in some central cells and along the hair cell lineage and is highest in progenitor cells undergoing differentiating divisions (purple arrow). J ccnd2a is more broadly expressed but is highest in the amplifying cell population (pink arrow) and absent from the hair cell lineage. K Heatmap of scaled gene expression across lateral line cell types during the averaged regeneration time course (Baek et al., 202212). L Heatmap of scaled gene expression during the regeneration time course. All genes are briefly upregulated at 0–30 min but show the largest activation between 3 − 10 h. atoh1a and ccndx show similar expression dynamics, whereas ccnd2a expression peaks slightly later. M Representative images of HCR in situ hybridization of ccndx (yellow) and ccnd2a (magenta) during the regeneration time course. Scale bar = 10 µm. Representative images of HCR in situ hybridization of ccndx (yellow) and atoh1a (magenta) during homeostasis (N) and 5 h after hair cell death (O). Scale bar = 10 µm.
A Representative image of a 5 dpf DAPI-stained zebrafish larva, posterior lateral line neuromast in boxed region. Scale bar = 200 µm. B Scanning electron micrograph of a 5 dpf zebrafish neuromast (dorsal view) with short stereocilia and long kinocilia in purple (adapted from Lush, M.E. and Piotrowski, T. (2014), Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn., 243: 1187-1202. https://doi.org/10.1002/dvdy.24167”10). C Transmission electron micrograph of a transverse section of a 5 dpf neuromast with hair cells in purple and mantle cells in blue. Additional support cells are unlabeled (adapted from Lush, M.E. and Piotrowski, T. (2014), Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn., 243: 1187-1202. https://doi.org/10.1002/dvdy.24167”10). Diagram of a neuromast showing a transverse section (D) and a dorsal view (E). Progenitor cells and hair cells are in purple, amplifying stem cells in the dorsal-ventral poles in pink and mantle cells in blue. F Integrated scRNA-seq UMAP plot of a neuromast regeneration time course (homeostasis, 0 min, 30 min, 1 h, 3 h, 5 h and 10 h after hair cell death; Baek et al., 202212). G–J scRNA-seq Feature Plots (Baek et al., 202212, https://piotrowskilab.shinyapps.io/neuromast_regeneration_scRNAseq_pub_2021/) illustrating gene-specific expression patterns. G pcna labels dividing, differentiating hair cell progenitors (purple arrow) and amplifying stem cells (pink arrow). H atoh1a is expressed in some central cells and marks the lineage from hair cell progenitors to hair cells (purple arrow). I ccndx is expressed in some central cells and along the hair cell lineage and is highest in progenitor cells undergoing differentiating divisions (purple arrow). J ccnd2a is more broadly expressed but is highest in the amplifying cell population (pink arrow) and absent from the hair cell lineage. K Heatmap of scaled gene expression across lateral line cell types during the averaged regeneration time course (Baek et al., 202212). L Heatmap of scaled gene expression during the regeneration time course. All genes are briefly upregulated at 0–30 min but show the largest activation between 3 − 10 h. atoh1a and ccndx show similar expression dynamics, whereas ccnd2a expression peaks slightly later. M Representative images of HCR in situ hybridization of ccndx (yellow) and ccnd2a (magenta) during the regeneration time course. Scale bar = 10 µm. Representative images of HCR in situ hybridization of ccndx (yellow) and atoh1a (magenta) during homeostasis (N) and 5 h after hair cell death (O). Scale bar = 10 µm.
Full size image
As in other mechanosensory organs in various species, zebrafish hair cell differentiation in neuromasts is negatively regulated via Notch-dependent lateral inhibition, and loss of Notch signaling causes the development of more hair cells at the expense of support cells15,17,18,21,22. In addition to its function in progenitor fate specification, Notch signaling also inhibits proliferation of differentiating progenitor cells during regeneration15,17. Therefore, during regeneration and immediately after hair cell death, Notch signaling is downregulated, leading to differentiation of progenitor cells, their proliferation and further differentiation into hair cells. The current belief is that cell proliferation is essential for neuromast hair cell regeneration, as cell cycle inhibition with pharmacological inhibitors leads to a failure in regeneration18,23.
Progenitor cell division produces a pair of hair cells with opposing polarity, ensuring the equal generation of hair cells that are sensitive to either rostrad or caudad water flow24. The transcription factor Emx2 determines hair cell polarity in both the lateral line and ear25,26,27,28,29,30. In neuromasts, it is expressed in only one of the two sibling hair cells, where it reverses the cell’s default anterior polarity. Notch-mediated lateral inhibition between the two initially equal progenitors inhibits the expression of Emx2 in one progenitor, and loss of Notch signaling causes both hair cells in the pair to acquire the same polarity25,28,30.
As proliferation is not only essential for life-long regeneration but also for correct hair cell polarity it is essential to elucidate how it is controlled. We previously characterized gene expression dynamics during regeneration in all neuromast cell types using single cell RNA-seq (scRNA-seq)11,12. Here we show that the proliferating amplifying stem cell and progenitor cell populations of the zebrafish lateral line express different cyclinD genes, which are G1-regulators that bind and activate cyclin-dependent kinases (CDK4/6) to initiate the cell cycle31,32. Amplifying cells express ccnd2a and dividing, differentiating progenitor cells express ccndx. Loss of ccndx causes lack of differentiating progenitor divisions while leaving amplifying divisions unaffected. Hair cells still regenerate in lower numbers, demonstrating that progenitor cell proliferation is not required for differentiation and regeneration of hair cells. In contrast, loss of ccnd2a only affects amplifying cell divisions, at least during development. ccnd2a driven by the ccndx promoter rescues progenitor proliferation in ccndx mutants, demonstrating that the effects of these two D-type cyclins are caused by their cell type-specific expression, not because they interact with different targets. Thus, proliferation in amplifying cells and differentiating progenitor cells is mechanistically uncoupled. We also show that Notch signaling inhibits ccndx during homeostasis and that the increase in hair cell progenitor proliferation after Notch downregulation during hair cell regeneration requires ccndx. Lastly, we demonstrate using scRNA-seq and functional analyses that loss of ccndx and progenitor proliferation lead to hair cell polarity defects due to hes2 downregulation and ectopic Emx2 expression. Our findings have important implications for the understanding of how proliferation of symmetrically dividing stem and progenitor cells is controlled during homeostasis and disease.
Results
Lateral line organs possess two populations of dividing cells with unique gene expression profiles
To uncover the mechanisms underlying hair cell regeneration, we set out to identify new regulators of this process. In zebrafish, the antibiotic neomycin induces rapid hair cell death followed by complete regeneration10,33,34. We used this approach in our previous scRNA-seq time course, where we identified all cell types of the regenerating neuromasts (Fig. 1F12,). With regard to dividing cells, two trajectories of proliferating populations were found to be marked by pcna expression (Fig. 1G and Supplementary Fig. 1A, B12,): the dividing hair cell progenitor cells mature into hair cells and are marked by the hair cell-specifying transcription factor atoh1a, whereas amplifying cells are defined as pcna+ cells that do not express atoh1a or hair cell genes (Fig. 1H12).To identify additional genes specific to either cell population, we queried our scRNA-seq data sets of 5 days post fertilization (dpf) neuromasts11,12. We found the cyclinD family member ccndx to be specifically expressed in differentiating progenitor cells (differentiating divisions, Fig. 1I, K), whereas ccnd2a was enriched in amplifying cells (amplifying divisions, Fig. 1J, K). Both cyclinD genes, as well as atoh1a and pcna were differentially expressed during regeneration with atoh1a and ccndx possessing similar expression dynamics (Fig. 1L and Supplementary Fig. 1C–F12,15,17,33. Upon neomycin treatment, all genes were initially downregulated followed by a strong upregulation between 3–10 h (hrs), when cells started to proliferate (Fig. 1L15). We validated the scRNA-seq time course data with hybridization chain reaction (HCR) mRNA expression analyses. ccndx and ccnd2a were lowly expressed during early stages of regeneration but showed strong upregulation at 5 h post hair cell death (Fig. 1M). As indicated by the scRNA-seq data ccndx was co-expressed with atoh1a in hair cell progenitors during homeostasis and 5 h after neomycin treatment (Fig. 1K, N, O). In contrast to ccndx, ccnd2a was enriched in amplifying cells during homeostasis, with broader expression 5–10 h after hair cell death (Fig. 1M and Supplementary Fig. 1D, H).
During development, ccndx and ccnd2a are both expressed in the 32 h post fertilization (hpf) migrating lateral line primordium (Supplementary Fig. 2A). As in mature neuromasts, ccndx is expressed in atoh1a-positive hair cell progenitor cells in the lateral line primordium and spinal cord neurons as described in the frog and zebrafish35,36. ccnd2a on the other hand is more broadly expressed in primordium cells but excluded from ccndx and atoh1a- expressing progenitor cells (Supplementary Fig. 2A). The exclusive expression of ccndx and ccnd2a is even more obvious in deposited, still developing neuromasts (Supplementary Fig. 2B). The stem cell status of support cells during development still needs to be determined. In conclusion, the two cyclinD genes are expressed in amplifying stem/support cells and proliferating hair cell progenitor cells, respectively, suggesting that proliferation is differentially regulated in stem cells/support cells and their differentiating progeny.
ccndx −/− neuromasts form new hair cells in the absence of proliferation
To test the function of ccndx, we generated a mutant using CRISPR/Cas9 (Supplementary Fig. 3A). The mutation introduces a stop codon, but truncated ccndx mRNA is still transcribed in these mutants (Supplementary Fig. 3F-G). 5 dpf ccndx−/− larvae looked grossly normal but lacked an inflated swim bladder and showed abnormal swimming behavior (Supplementary Fig. 3B-C). The mutant larvae failed to inflate their swim bladder and were not viable. Nevertheless, the migrating lateral line primordium deposited neuromasts normally during embryogenesis (Supplementary Fig. 3D-E). Previous morpholino-induced knockdown of ccndx in zebrafish and frogs resulted in defects in motor neuron formation and axonal outgrowth35,36, however anti-acetylated tubulin staining in 5 dpf ccndx−/− larvae showed no obvious differences in motor axon outgrowth (Supplementary Fig. 3H–K). The morpholino-induced phenotype could be more severe than the mutant phenotype as the morpholino also blocks maternal mRNA, or alternatively, it causes off target effects. To test the role of ccndx in hair cell formation, we analyzed a hair cell-specific reporter line (Tg(myo6b:H2B-mScarlet-I)) in ccndx mutants. We observed a reduced number of hair cells in 5 dpf homeostatic ccndx−/− neuromasts and fewer regenerated hair cells 24- and 48 hrs after neomycin-induced hair cell death compared to sibling larvae (Fig. 2A–C). As mutant larvae die soon after, we were not able to assess if mutant hair cell numbers eventually catch up with sibling hair cell numbers.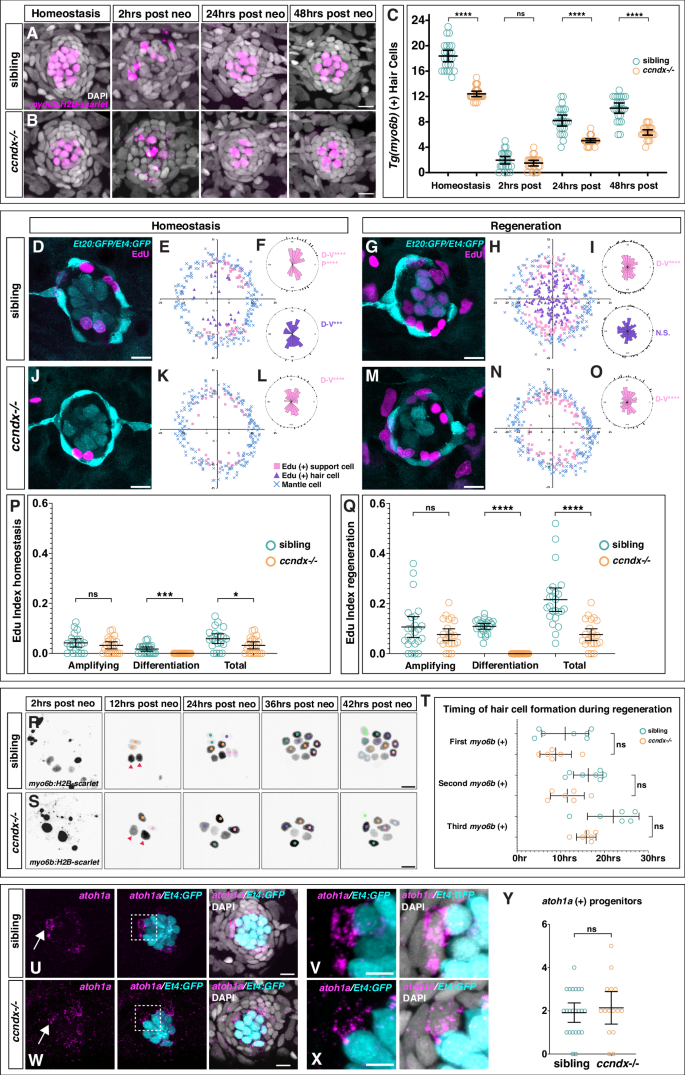 Time course of hair cell regeneration in sibling (A) and ccndx−/− (B) at homeostasis and 2, 24 or 48 h after hair cell death. Hair cells are labeled by myo6b:H2B-mScarlet-I (magenta). Scale bar = 10 µm. C Hair cell counts in sibling and ccndx−/− at homeostasis and 2, 24 or 48 hrs after hair cell death
Time course of hair cell regeneration in sibling (A) and ccndx−/− (B) at homeostasis and 2, 24 or 48 h after hair cell death. Hair cells are labeled by myo6b:H2B-mScarlet-I (magenta). Scale bar = 10 µm. C Hair cell counts in sibling and ccndx−/− at homeostasis and 2, 24 or 48 hrs after hair cell deathList of Referenes
- Mark E. Lush, Ya-Yin Tsai, Shiyuan Chen, Daniela Münch, Julia Peloggia, Jeremy E. Sandler, Tatjana Piotrowski. Stem and progenitor cell proliferation are independently regulated by cell type-specific cyclinD genes. Nature Communications, 2025; 16 (1) DOI: 10.1038/s41467-025-60251-0
Cite This Article as
No tags found for this post