We reach more than 65,000 registered users in Dec!! Register Now
Study reveals structure of crucial receptor in brain development, function
- September 23, 2023
- 7 Views
- 0 Likes
- 0 Comment
OHSU scientists elucidate structure of receptors targeted by antidepressants, other pharmaceutical drugs; could lead to improved therapies
Scientists have revealed the molecular structure of a type of receptor that’s crucial to brain development and function.
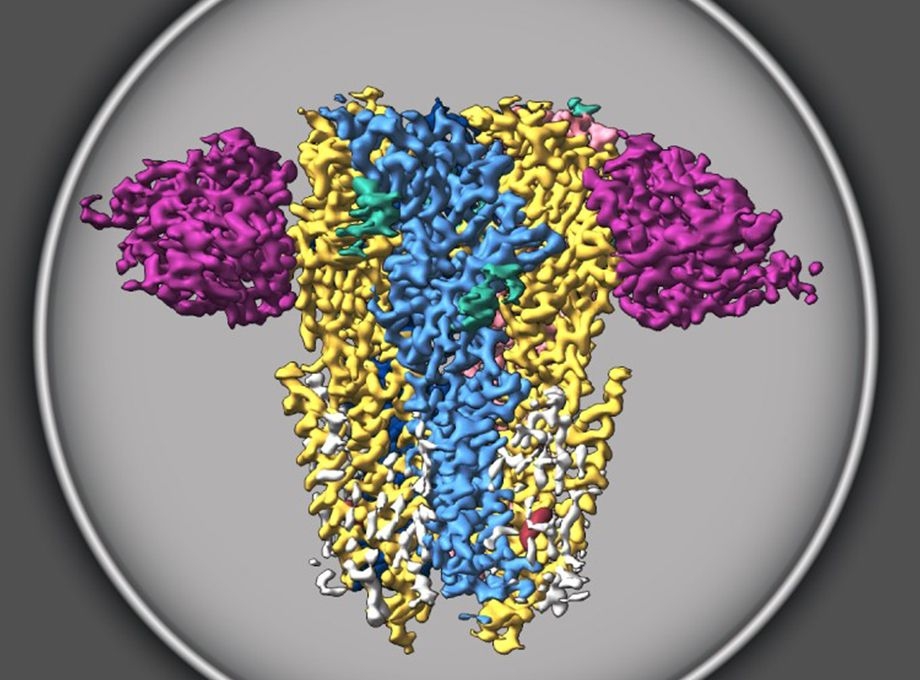
Known as Type A GABA receptors, these receptors are already targeted by pharmaceutical anesthetics, sedatives and antidepressants because of their important role in brain function. The discovery, published today in the journal Nature, reveals the dominant assemblies and states of the GABA receptor, a finding that could enable the development of new compounds that more specifically target a range of medical disorders.
“It is the main player that balances excitation and inhibition in the brain,” said lead author Chang Sun, Ph.D., a postdoctoral researcher in the Vollum Institute at Oregon Health & Science University. “It affects all aspects of brain function, from motor function, to memory and learning, and also emotion and anxiety.” Eric Gouaux, Ph.D. (OHSU)“Because the off switch is so crucial, GABA receptors are spread throughout the entire brain,” added senior author Eric Gouaux, Ph.D., senior scientist in OHSU’s Vollum Institute and an investigator with the Howard Hughes Medical Institute.
Eric Gouaux, Ph.D. (OHSU)“Because the off switch is so crucial, GABA receptors are spread throughout the entire brain,” added senior author Eric Gouaux, Ph.D., senior scientist in OHSU’s Vollum Institute and an investigator with the Howard Hughes Medical Institute.
The receptor is defined by five-sided, or pentameric, assemblies derived from 19 different subunits, each of which give rise to a vast number of configurations that may or may not be clinically relevant. In this case, researchers painstakingly isolated native assemblies from mice and then infused them with common medications used to treat insomnia and postpartum depression.
They were then able to visualize three major structural populations of the receptor.
“This study shows the dominant assemblies and states of the GABA receptor,” Gouaux said. “That’s really the huge breakthrough — nobody had been able to figure out which of the hundreds of thousands of these assemblies are most highly populated.”
The discovery shows the GABA receptor in its native state as opposed to tissue culture, as demonstrated in previous work, said co-author Sarah Clark, Ph.D., a former postdoctoral fellow in the Gouaux lab and now an assistant professor at Oregon State University. Researchers leveraged state-of-the-art cryogenic electron microscopy to reveal the structure in its natural state, rather than earlier techniques that required crystallizing vast quantities of identical molecules to form an artificial picture of their native structure.
“We used a combination of cryo-EM as well as single-molecule microscopy technique, which allowed us to count the subunits in each pentameric complex,” she said.
Gouaux credited OHSU, as well as the Jennifer and Bernard Lacroute Endowed Chair in Neuroscience, for supporting this high-risk, high-reward research, along with the Howard Hughes Medical Institute, for providing the sustained support over more than three years to generate the discovery.
“This kind of work is difficult to fund because no one thinks it will work,” Gouaux said.
Funding support was provided by the National Institutes of Health, award 5R01GM10040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
All research involving animal subjects at OHSU must be reviewed and approved by the university’s Institutional Animal Care and Use Committee (IACUC). The IACUC’s priority is to ensure the health and safety of animal research subjects. The IACUC also reviews procedures to ensure the health and safety of the people who work with the animals. No live animal work may be conducted at OHSU without IACUC approval.
List of Referenes
- Chang Sun, Hongtao Zhu, Sarah Clark, Eric Gouaux. Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology. Nature, 2023; DOI: 10.1038/s41586-023-06556-w
Cite This Article as
No tags found for this post