We reach more than 65,000 registered users in Dec!! Register Now
Successful Development of a Perfect Diamagnetic Conducting Polymer
- December 22, 2024
- 2 Views
- 0 Likes
- 0 Comment
Conducting polymers exhibit a variety of properties in addition to their conductivity. Research has explored their use in light-emitting devices, electromagnetic wave shielding, and anticorrosion materials. One notable characteristic is paramagnetism.
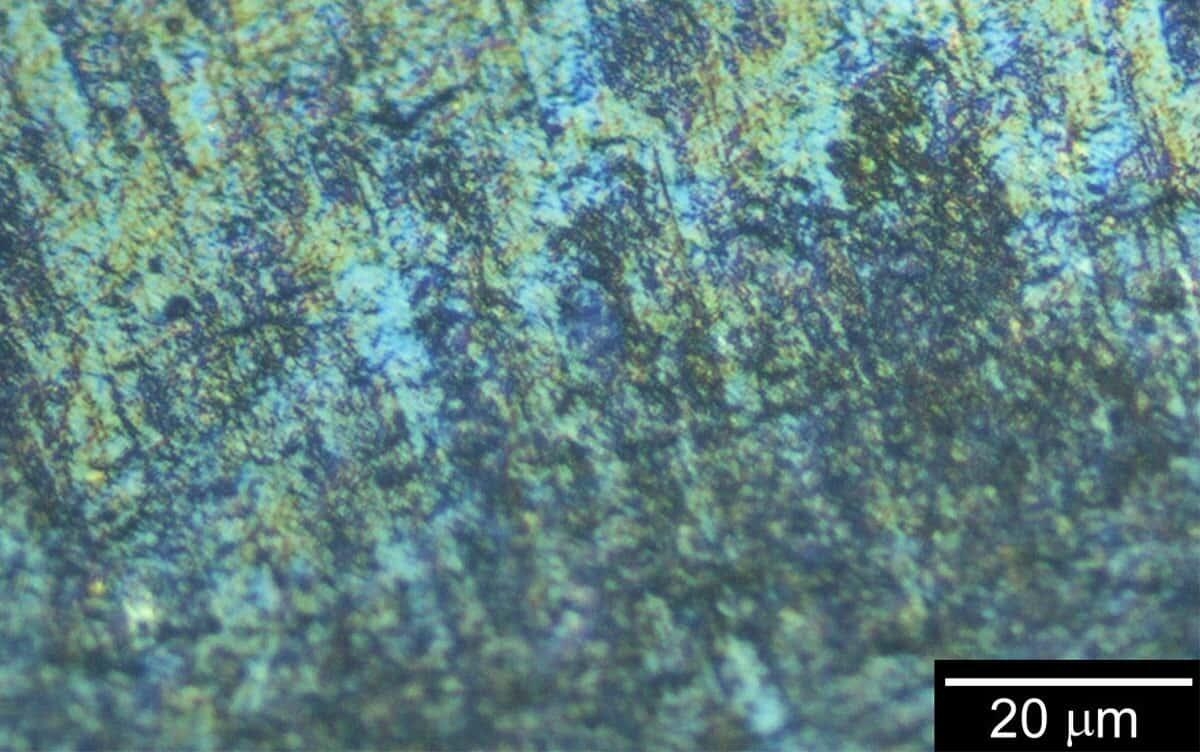
The research team has previously developed methods for synthesizing various conducting polymers. In this study, they successfully synthesized polyaniline, one of the most commonly studied conductive polymers, in the presence of iron sulfate, imparting perfect diamagnetism—a property that excludes external magnetic fields from the material. This behavior is analogous to that of superconductors and contrasts with the paramagnetism typically found in conducting polymers.
Superconducting Quantum Interference Device measurements of the synthesized polyaniline confirmed that its magnetic susceptibility exhibits a gradual negative shift from approximately 100 K (−173°C), and it demonstrates perfect antiferromagnetism below 24 K (−249°C). Conducting polymers, which are also organic semiconductors, usually exhibit strong temperature dependence in their electrical conductivity. At lower temperatures, their conductivity typically decreases, while electrical resistance increases. However, the polyaniline synthesized in this study showed minimal variation in electrical resistance with temperature. A significant reduction in electrical conductivity was only observed at extremely low temperatures.
The discovery of perfect diamagnetism in polyaniline represents a unique phenomenon not observed in conventional organic or inorganic conductive materials. It is plausible that an unconventional mechanism of perfect diamagnetism is at play, potentially leading to novel advancements in the field of conductive polymers.
List of Referenes
- Hiromasa Goto, Ryo Miyashita. Perfect Diamagnetism of Polyaniline. The Journal of Physical Chemistry B, 2024; 128 (40): 9917 DOI: 10.1021/acs.jpcb.4c05317
Cite This Article as
No tags found for this post